Study Manager
ObvioHealth is a Clinical Research Organization (CRO) that conducts clinical trials to validate pharmaceutical claims, helping drug companies secure FDA approval.
Highlights 📌
Zero to One, React Desktop app, Strategy & Vision, Design System, Third party Integrations.
Team 🦸🏻♀️🦹🏼♀️🦸🏽♂️
CTO, Senior Product Manager, Director of Development, Director of Product, Director of Design, Sales team, Operations Lead
Study Manager is a remote patient monitoring system that enables ObvioHealth to efficiently conduct and manage clinical studies.
Timeline 🗓️
~ 2 years
(Agile development cycle)
Role 👩🏻💻
As the owner of the Study Manager product vertical, I led its design, research, vision, strategy, and development. I worked closely with executive leadership, product managers, developers, and design peers to bring the product to life.
Current System
ObvioHealth managed study participants and data through a vendor application, which served as a temporary solution despite its inefficiencies, poor user experience, and restrictive capabilities.
Challenge
The need to replace the vendor application became even more urgent when a new client required a complex study that the existing system couldn’t support.
How might we
Deliver a scalable system that enables ObvioHealth to run and manage complex clinical studies successfully.
Solution
ACTION ORIENTED
Interactive Data Viz
To empower users to complete tasks efficiently, I implemented interactive, actionable data visualizations that present the right data at the right time for better decision-making.
COLLABORATION
Notes Panel
To enhance seamless collaboration and keep teams aligned, I introduced the Notes Panel, allowing study managers to track all the actions taken by their peers in the Participant Details Hub.
DATA-FRIENDLY
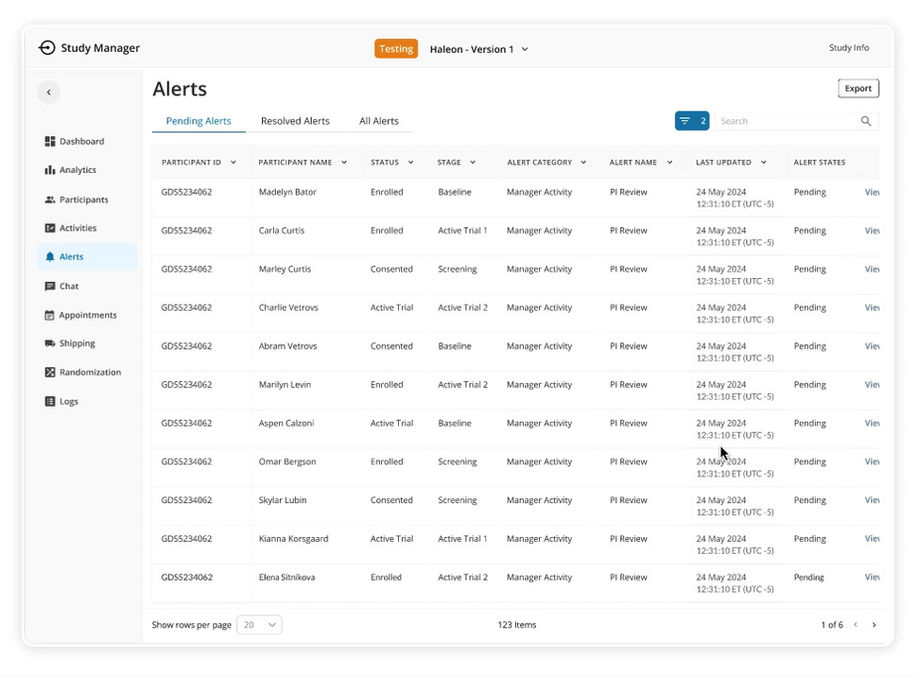
Tables
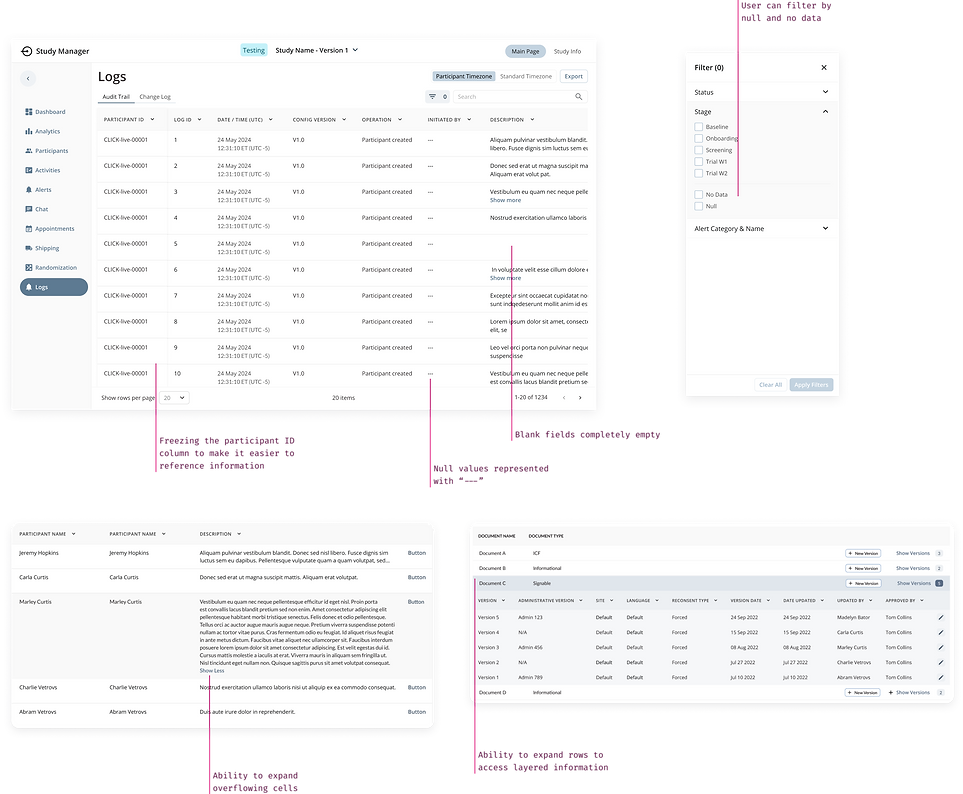
One of the biggest pain points for users was dealing with an overwhelming volume of data, making it inaccessible and unapproachable. To address this, I implemented Smart Tables with collapsible rows and cells, along with a robust Search & Filter panel, creating a more intuitive and user-friendly experience.
Impact

Persona
I interviewed 10 Study Managers or COACH (Clinical Oversight and Coordination Hub) as we called them. I also interviewed operational heads from 5 clinical sites to understand user needs and challenges
User Insights

Clinical Study Audit
As part of research, I audited the existing clinical study protocols to better understand the content and processes.


Information Architecture
Facilitated a 2 day design workshop with key stakeholders and executives to create a system map of the Study Manager application
Design System
Built the design system from scratch along with patterns and guidance.